Abstract

BACKGROUND: Protein S (ProS) is a key negative regulator of the coagulation cascade. ProS exists in the circulation either free or in complex with C4b binding protein (C4bBP) of which the free form serves as a cofactor to enhance anticoagulant activity of tissue factor pathway inhibitor alpha (TFPIα) and activated protein C (aPC). ProS deletion has been shown to restore hemostasis in a hemophilia A mouse model suggesting that ProS inhibition may be a viable approach to treat various bleeding disorders. VGA039 is a monoclonal antibody directed against human ProS that inhibits ProS cofactor activity for TFPIα and aPC, thus enhancing thrombin generation by acting on both the initiation (TFPIα) and propagation (aPC) phases of coagulation.
Current treatments for congenital factor deficiencies involve factor replacement therapies. However, due to relatively quick turnover, factor replacement is onerous with poor patient compliance. VGA039, as a subcutaneously bioavailable universal hemostatic agent, could reduce treatment burden in various congenital factor deficiencies (e.g. von Willebrand Disease, vWD).
OBJECTIVES: The objectives were to characterize the molecular pharmacology of VGA039 and to evaluate the pharmacokinetic (PK) profile and pharmacodynamic (PD) effects of VGA039 in non-human primates (NHPs).
RESULTS: VGA039 was selected from an immunization campaign using human ProS, where cross-reactivity with human and NHP ProS and the reversal of aPC and TFPIα anticoagulant activities were criteria for selection. VGA039 bound to human and NHP, but not porcine, canine or rodent ProS. In vitro, thrombin generation assays (TGA) demonstrated VGA039 increased thrombin generation in aPC-treated normal human and NHP plasma with an EC50 of ~10 µg/mL. Similarly, in pooled normal human plasma containing exogenous recombinant human TFPIα (rTFPIα), VGA039 enhanced tissue factor induced thrombin generation in a concentration-dependent manner with an EC50 of ~10 µg/mL.
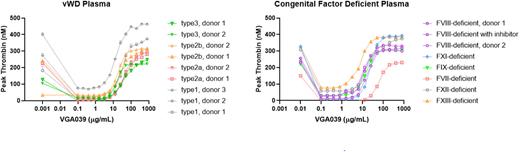
The ability of VGA039 to restore thrombin generation and clot formation in various bleeding disorders was assessed by TGA and microfluidics assays using either congenital factor deficient patient plasma or normal whole blood pharmacologically treated to mimic specific factor deficiencies. VGA039 increased thrombin generation in a concentration dependent manner in congenital vWD, FVII-, FVIII-, FIX-, FXI-, and FXIII-deficient plasma (Figure), but not in FX- or FV-deficient plasma (not shown) in the presence of aPC. Additionally, VGA039 increased fibrin deposition in a collagen-coated microfluidics chamber using recalcified normal blood containing a neutralizing anti-FVIII antibody. These results support the interpretation that VGA039 may be able to effectively compensate for the absence of coagulation factors to improve coagulation.
In vivo, VGA039 administration either intravenously (IV) or subcutaneously (SC) showed dose-proportional exposure without evidence of target-mediated drug disposition. VGA039 was relatively long-lived (t1/2 of 21 days IV and 12 days SC administrations at 1 mg/kg) and showed dose-dependent accumulation with repeat dosing. Ex vivo PD assessment of samples from dosed animals demonstrated a concentration dependent increase in thrombin generation between 10 µg/mL and 100 µg/mL [VGA039]plasma. Consistent with these results, VGA039 increased plasma D-dimer concentrations in a dose- and concentration-dependent manner. After IV (≥3 mg/kg) or SC (≥10 mg/kg) single-dose administration of VGA039, plasma D-dimer concentrations were generally greater than control or baseline values when plasma VGA039 concentrations were ≥10 μg/mL, with maximal D-dimer concentrations leveling off at a mean of ~2 µg/mL and returning to pre-dose values following VGA039 clearance. These data demonstrate that VGA039 is subcutaneously bioavailable and promotes thrombin generation in vivo.
CONCLUSION: Our results indicate that VGA039 is a novel, pro-hemostatic monoclonal antibody that inhibits the cofactor activity of ProS. Due to its mechanism of action, subcutaneous bioavailability and long half-life, VGA039 has the potential to be a universal pro-hemostatic agent that can ease the treatment burden for patients on factor replacement therapy.
Disclosures
Leong:Star Therapeutics: Current Employment. Byun:Star Therapeutics: Current Employment. Kim:BioMarin Pharmaceutical: Ended employment in the past 24 months; Catalyst Biosciences: Ended employment in the past 24 months; Star Therapeutics: Current Employment; Electra Therapeutics: Current Employment. Huck:Electra Therapeutics: Current Employment; Star Therapeutics: Current Employment. Silva:Star Therapeutics: Ended employment in the past 24 months. Chan:Electra Therapeutics: Current Employment; Star Therapeutics: Current Employment. Cho:Star Therapeutics: Current Employment. Liu:Star Therapeutics: Current Employment; Electra Therapeutics: Current Employment. Horvath:Bluebird bio: Current Employment; Electra Therapeutics: Current Employment; Star Therapeutics: Current Employment. Moore:Electra Therapeutics: Current Employment; Star Therapeutics: Current Employment. Panicker:Electra Therapeutics: Current Employment; Star Therapeutics: Current Employment.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.